Cyanobacterial Light Harvesting and Photoprotection
Cyanobacteria are most commonly green or blue-green in appearance, and their color can be attributed to the most abundant pigments found in their cells. Pigments are molecules that absorb visible light, and they are often bound to proteins inside the cell.
The most common light-absorbing pigments in cyanobacteria are usually blue “bilin” molecules and green chlorophyll molecules, both of which harvest energy from sunlight in order to photosynthetically convert it to other useful forms of energy (i.e. chemical energy). Blue bilin pigments are found in particularly high concentrations in cyanobacteria because they are part of an important light-harvesting ‘antenna’ in these organisms: the phycobilisome. The phycobilisome antenna efficiently gathers energy from light and transfers this energy to other proteins involved in photosynthesis.
|
|
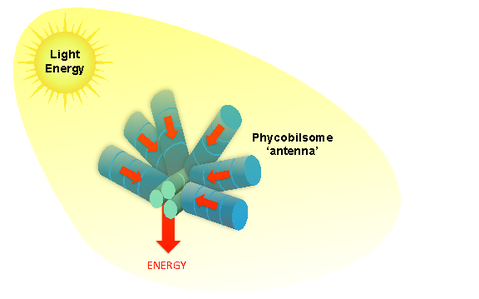
A simplified illustration of the light harvesting protein ‘antenna’ of cyanobacteria, the phycobilisome. Energy absorbed by blue bilin pigments in the rods is funneled to the core of the structure where it can be efficiently transferred to other proteins involved in photosynthesis. (Image Credit: Ryan Leverenz and Cheryl Kerfeld)
|
While the bilin pigments of the phycobilisome and the chlorophyll pigments found in other photosynthetic proteins are the most abundant pigments in cyanobacteria, there are other pigments and pigment-protein complexes which perform critical functions in cyanobacterial cells. The presence of less-abundant pigments becomes more apparent when we break open the cells, extract their proteins, and then separate these proteins based on their specific characteristics (i.e. their size, charge, and solubility). The separation of water-soluble proteins from the extract of a cyanobacterium [Arthrospira platensis or “Spirulina”] can be seen in the pictures/movies below.
|
Purification of pigment-binding proteins. The water-soluble proteins of cyanobacteria can be easily separated using column chromatography (Left). The separated proteins (right) exhibit a wide variety of colors due to the different pigments that they bind. (Image Credit: Ryan Leverenz and Cheryl Kerfeld)
|
Certain pigmented proteins function as light-activated “switches”- triggering biological functions in direct response to the color or intensity of light to which the cell/protein is exposed. The need for cyanobacteria to have functions directly triggered by light is not surprising, considering that sunlight is the primary source of energy for most cyanobacteria. While cyanobacteria don’t “see” in the same way that human beings and other animals do (using pigmented proteins in the retina of the eyeball to spot a good meal!), they still must sense and respond to light in order to adjust to changes in their environment and find the energy they need to grow and multiply. Cyanobacteria use light-activated protein switches to optimize their exposure and response to sunlight through, for instance, light-triggered movement or light-triggered regulation of photosynthesis.
Our lab is particularly interested in a light-activated protein responsible for regulating photosynthesis in cyanobacteria – the Orange Carotenoid Protein (OCP), discovered in 1981 by David Krogmann (1934-2016). Intense light activates this protein in a striking fashion, changing the appearance from orange to red.
Our lab is particularly interested in a light-activated protein responsible for regulating photosynthesis in cyanobacteria – the Orange Carotenoid Protein (OCP), discovered in 1981 by David Krogmann (1934-2016). Intense light activates this protein in a striking fashion, changing the appearance from orange to red.
The light-activated red form of the protein performs a critical function in the cell – it binds directly to the phycobilisome antenna and effectively short-circuits energy flow by dissipating energy harmlessly as heat.
|
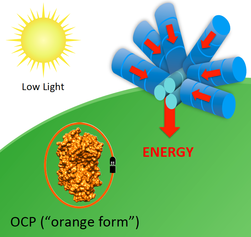
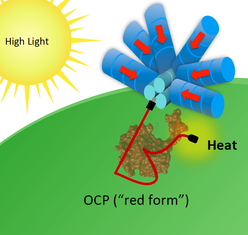
Illustrations of OCP-dependent dissipation of energy absorbed by the phycobilisome. In low light the OCP remains in its orange form and energy is funneled by the phycobilisome in its normal fashion (left). In high light (right), the OCP is activated to its active red form, which binds to the phycobilisome and dissipates energy harmlessly as heat. (Image Credit: Ryan Leverenz and Cheryl Kerfeld)
|
By short-circuiting energy flow from the antenna, the OCP can protect the cell from potentially harmful effects of excess light. These hazards include the generation of powerful ‘oxidants’ that can accumulate under high light conditions and damage the cell’s photosynthetic machinery when too much energy is absorbed from sunlight. The OCP plays a pre-emptive role in avoiding the formation of these oxidants (by dissipating energy from an over-excited antenna), and may also directly neutralize certain harmful oxidants through ‘anti-oxidant’ chemical properties of its bound carotenoid.
The OCP is the only light-activated protein switch known to use a carotenoid pigment to drive its light-dependent function. The mechanism by which the OCP is activated by light, binds to the phycobilisome, and dissipates energy must be elucidated in order to understand how the OCP performs its photoprotective function, and this remains an active area of research in our laboratory.
-Ryan Leverenz and Matt Melnicki
The OCP is the only light-activated protein switch known to use a carotenoid pigment to drive its light-dependent function. The mechanism by which the OCP is activated by light, binds to the phycobilisome, and dissipates energy must be elucidated in order to understand how the OCP performs its photoprotective function, and this remains an active area of research in our laboratory.
-Ryan Leverenz and Matt Melnicki

This work by Ryan Leverenz, Matt Melnicki, and Cheryl Kerfeld is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.